The results of some calculations just need to be showcased.
This ab initio chemical reaction simulation took a huge amount of time and effort to generate.
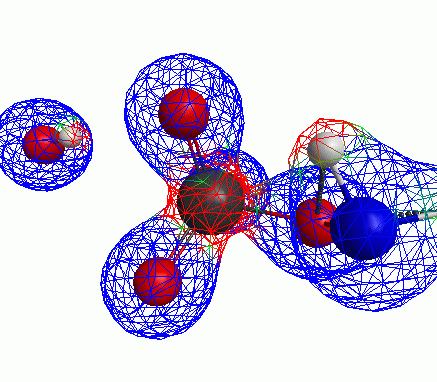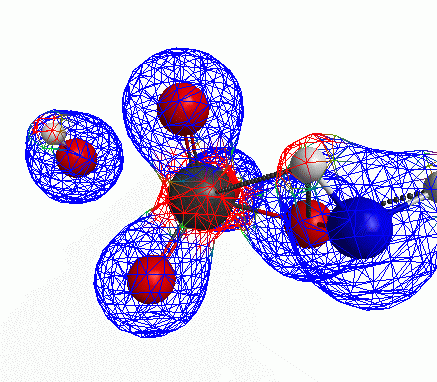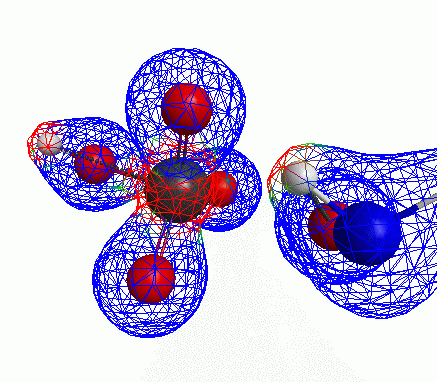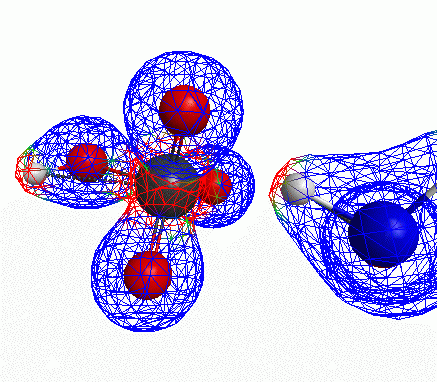
Depicted here is an attack by hydroxide (-1 charge) on aminophosphate (-2 charge). The black ball is phosphorous, red is oxygen, white is hydrogen and blue is nitrogen. As should be evident by my usual mode of operation, the surface is an equipotential surface containing 90% of all electron density. The coloration of that surface is in electrostatic potential with blue as negative and red as positive. Dashed lines are hydrogen bonds, single sticks are single covalent bonds and double sticks are double covalent bonds. The reaction produces singly protonated phosphate (-2 charge) and deprotonated hydroxyamine (-1 charge) . A polarizable continuum model is in use in the simulation to place the reaction effectively in water (you need this to help dampen the repulsive forces charged portions of these molecules have on each other.) This structure was calculated using the Pople 6-31G** basis set, literally at the limit of my computer, in order to include some polarizability in the atoms and to create enough variational flexibility in the diffuse region of the model to pick up the interactions between the molecules approaching each other. This basis set is very big and costly, meaning that I can only do fairly small systems with it on my computer using this strategy.
I’ve been trying to find a version of this particular reaction for literally months now, hampered in large part by my lack of skill with ab initio and by my sometimes shaky knowledge of organic chemistry. The last reaction that I posted with phosphate and water was a biproduct of my search for this reaction. This particular reaction is one possible prototype for the reaction by which DNA is polymerized from dNTPs. In this case, the hydroxide is standing in for the 3′ hydroxyl of oligomeric DNA and the hydroxylamine is standing in for pyrophosphate. The reaction is a form of transesterification reaction. In DNA, the resulting products would be phosphodiester linkages between nucleotide bases.
To make this reaction proceed, a deprotonated hydroxyl must attack through the face of tetrahedral phosphate opposite the desired leaving group. Further, the attacking group must be a poorer leaving group than the desired group –I had this problem early in my search. For the search to work, I needed to guess the appropriate transition state, which I didn’t think too clearly about until I’d already wrapped four or five failures under my belt. As it turns out, the phosphorous must go from tetrahedral valence to a trigonal-bipyrimidal state. I then needed to tease out spurious other “reactions” including a rotation by the hydroxyamine. This becomes difficult with more complicated molecules because additional degrees of freedom allow for a lot of unimportant motion that can totally screw up the search.
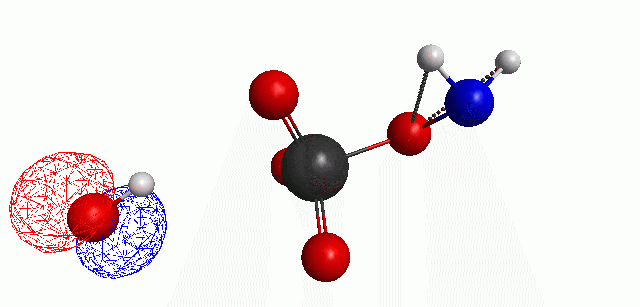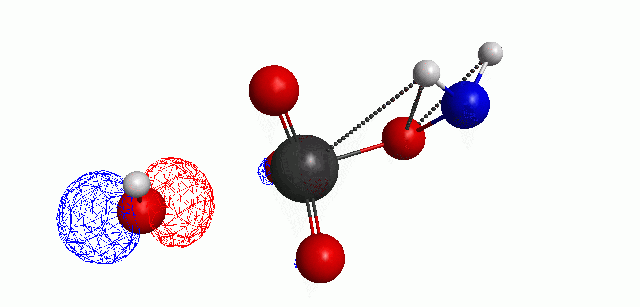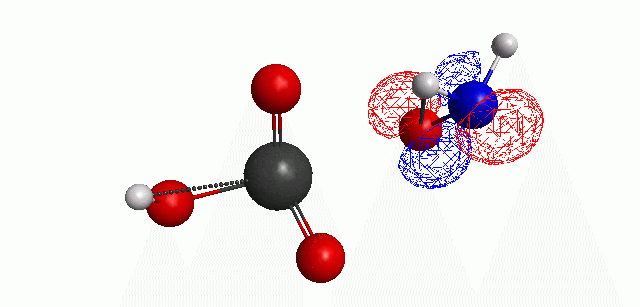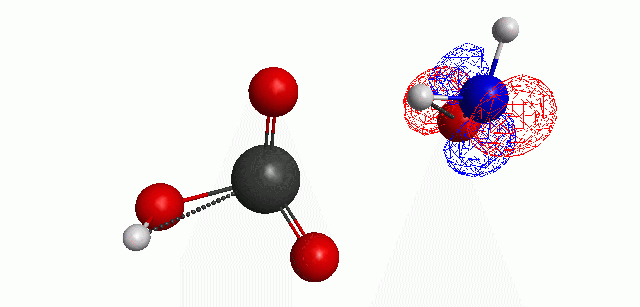
The highest occupied molecular orbital (HOMO) throughout this reaction is, I think, very interesting.
When these chemicals are separated in exclusion of one another, the polarization of the water continuum allows the molecular orbitals to have negative energy, meaning that they can stay occupied (theoretically, if you believe the MO energies, which are known to be off). But, as the hydroxide is placed near the aminophosphate, the orbitals, particularly the HOMO, shift to positive energy, meaning that the hydroxide wants to give its electrons up. You can then see the lobe of the hydroxide HOMO rotate into the open region of the phosphate tetrahedron during the attack whereby the HOMO is transferred to the hydroxyamine, which is then displaced. This new HOMO still has positive energy and, even though it actually starts to rotate to face the phosphate, the potential energy surface of the reaction, meaning the charge forces and orbital exclusion, force the potential reactants apart. The hydroxide HOMO peaks at 0.238 Hartrees, while the Hydroxyamine peaks at 0.243 Hartrees. I would actually sort of think that hydroxyamine would be the better attacker, but I also was aware that the nitrogen-based leave group should be better than the hydrogen in hydroxide and would give me a better chance at finding this reaction. To be clear, the hydroxyamine HOMO is definitely antibonding (just look at it) and this molecule would still be reactive elsewhere.
(Quick edit 7-1-19: Also looking at the HOMO, you can see a point where the HOMO spreads from hydroxide over onto the phosphate just before the reaction has “officially” taken place by reaching the transition state. This means that electrons from the hydroxide have leaked over onto the phosphate and have mixed with the electrons already present on the oxygens located there. This comes back to the Born-Oppenheimer approximation; because of the difference in masses, the time scale governing the motion of the electrons is very different from that of the nuclei. When the HOMO has shown up on the phosphorous, it means that the electrons are tunneling over and back even as the nucleus still moves in.)
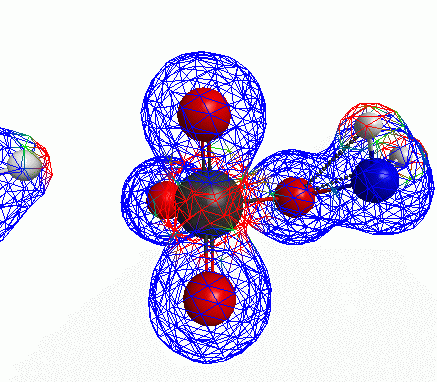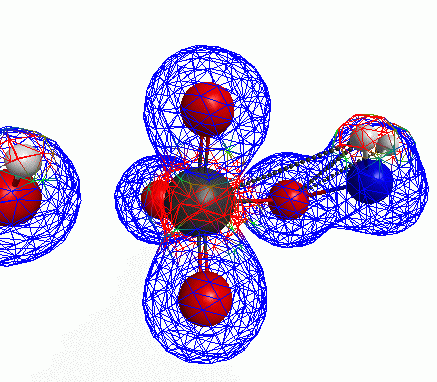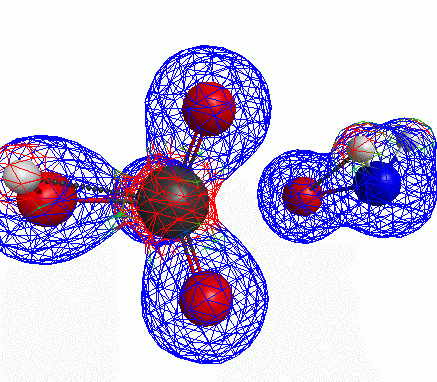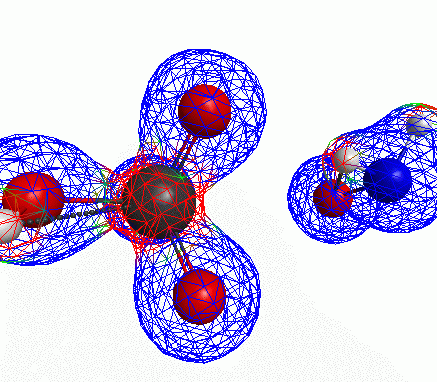
Just some other views of the same thing.
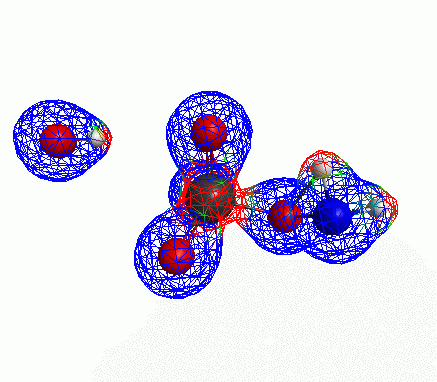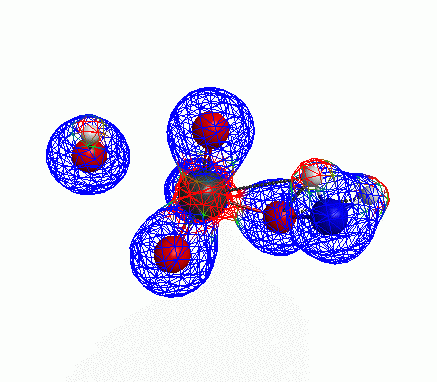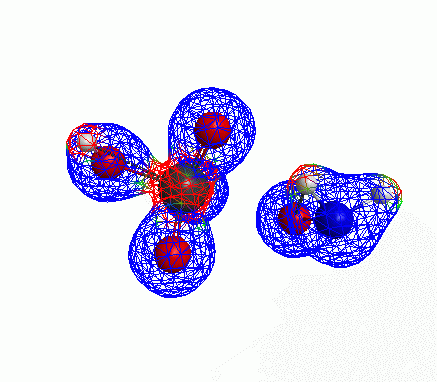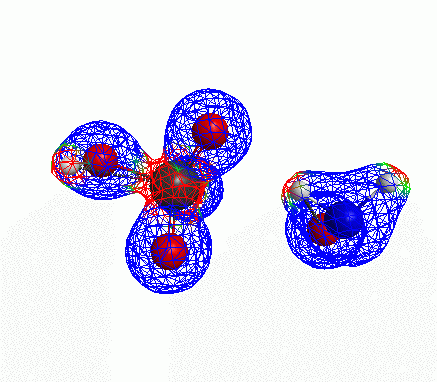
Most of my reason for doing this post was to just add some kind of pretty pictures. You can’t blame me; this took a ridiculous amount of time to produce and I feel like showing it.
